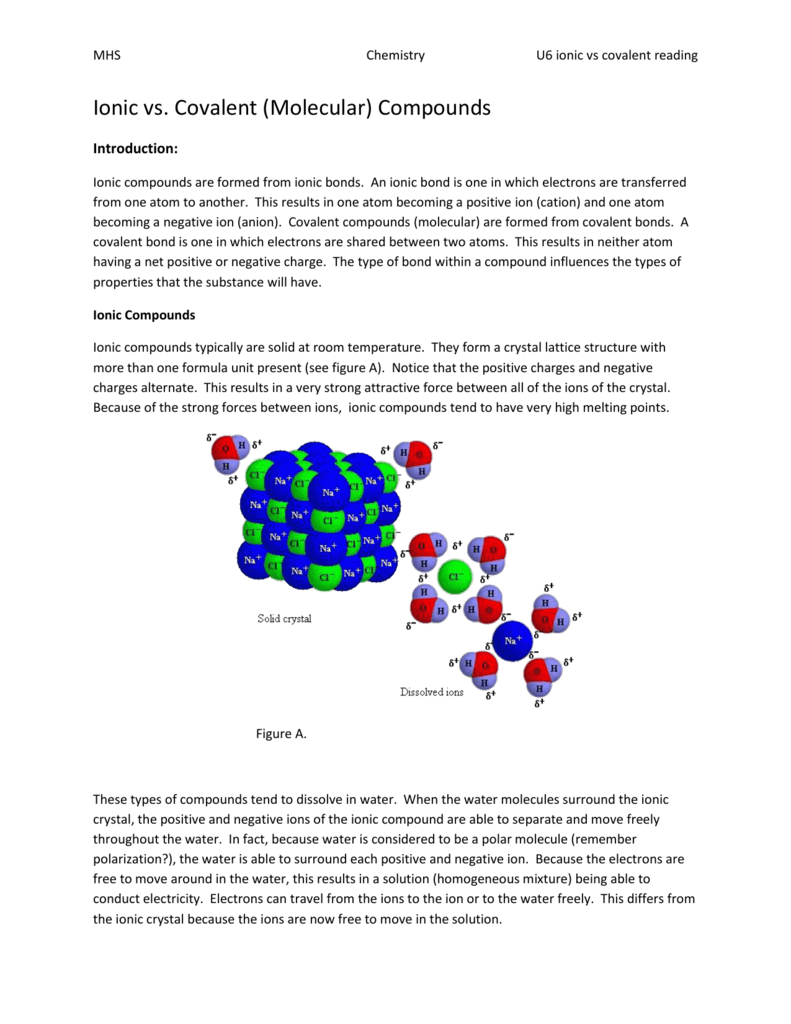
It forms a giant lattice due to the strong ionic force of attraction between the similar molecules NaCl - NaCl attraction is strong so they require huge amount of energyat higher temperature to break those forces. Although solid ionic compounds do not conduct electricity because there are no free mobile ions or electrons ionic compounds dissolved in water make an electrically conductive solution.
This explains why many of them are liquids or gases at room temperature.

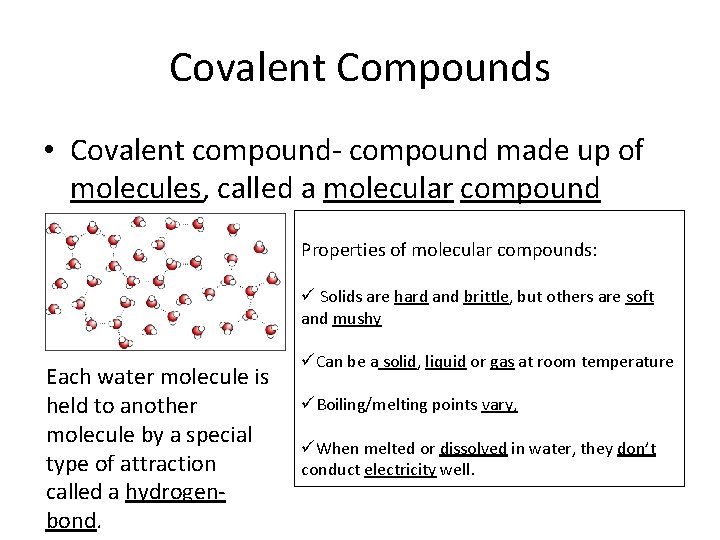
. Corn starch one of the covalent compounds is solid at room temperature. These compounds are very different from ionic compounds like sodium chloride NaCl. While covalent bonds between atoms are quite strong but attractions between moleculescompounds or intermolecular forces are relatively weak.
Most ionic compounds have lower boiling points than most molecular compounds. Molecular compounds of low molecular weight tend to be gases at room temperature. These are a result of poor coordination between the ions in solid form.
4 covalent -164 Nitrogen oxide NO covalent -152 Sodium chloride NaCl ionic 1413 Lithium fluoride LiF ionic 1676 Q. It depends on the compound. Which of the following is most likely not a gas at room temperature.
Uses of covalent compounds as solvents. Which of the following statements best explains why all ionic compounds are solids at room temperature whereas many molecular compounds are not. B Among the covalent compounds those that are relatively nonpolar and have low molecular masses are.
How do you know the state of a compound at room temperature. So the hypothesis was mostly supported except for this one data point. Which two properties are more typical of molecular compounds than of ionic compounds1.
Benzene melts at 6C and boils at 80C. Hence they exist as liquids at room temperature and are volatile. At room temperature and pressure there are gaseous liquid andsolid molecular covalent compounds.
With only rare exception these gases have relatively small molecular weights. Glucose and table sugar are solid at room. Because ionic compounds are formed by alternating positive and negative ions and because they are all held together with these strong electrostatic forces the oppositely charged ions pack tightly with each other and form a crystal lattice structure that is extremely hard to break.
Carbon dioxide and methane are gases at room temperature. Ionic compounds are formed when metal atoms lose one or more of their. - They can be solids at room temperature.
Molecular Compounds can be in any state solid liquid or gas at room temperature whereas Ionic Compounds are always in solid state with forming crystalline appearance. At room temperature and normal atmospheric pressure covalent compounds may exist as a solid a liquid or a gas whereas ionic compounds exist only as solids. Methane CH4 ethylene C2H4Liquid benzene C6H6 ethanol C2H5OHSolid.
Chlorine Cl2 is a gas at room temperature but sodium chlorideNaCl is a solid at room temperature. Molecular Compounds have low melting and boiling point as compare to the Ionic Compounds. What is the meaning of molecular compound.
Covalent compounds in the form of liquids are mostly used as solvents in our daily life. Many covalent compounds have low melting and boiling points. Name of CompoundChemical For- mula Type of Compound Boiling Point C Methane CH.
The two covalent compounds in the table are gases at room temperature which is 20C. Typically they involve ions with relatively complex organic components. Thus simple molecular compounds have very low melting and boiling points.
Most of these liquids are organic compounds. An ionic compound is most likely a solid at room temperature and pressure whereas a covalent compound may be a solid a liquid or a gas. They are gases or liquids at room temperature2.
If the normal melting point of a substance is below room temperature the substance is a liquid at room temperature. These compounds are found in all three physical states at room temperature. Covalent compounds needed very less amount of energy.
Which of the following is most likely not a gas at room temperature. In general covalent compounds are all gases at room temperature. Molecular Compounds are poor conductor of electricity whereas Ionic Compounds are good.
The property of being solid is more common to ionic compounds. Solids do not conduct electricity but liquids do4. Intermolecular forces are much weaker then ionic bonds metallic bonds and covalent bonds.
Ionic compounds at room temperature usually exist as solids with distinct crystalline lattice structures. Ionic vs Covalent Bonds Summary Ionic Bonds Covalent Bonds Boiling Point High Low State at Room Temperature Solid Liquid or Gas Examples Sodium chloride NaCl Sulfuric Acid H 2 SO 4 Methane CH 4 Hydrochloric acid HCl Chemical Species Metal and nometal remember hydrogen can act either way Two nonmetals. Arrange the statements from top to bottom to describe general process for naming binary molecular compounds when given the formula.
H2 E CH The apparatus pictured below is used to conduct the following experiment. Molecular compounds of low molecular weight tend to be gases at room temperature. The particles of ionic compounds are held together by stronger nuclear forces than the particles of molecular.
They have high melting points3. Thus at normal room temperatures the strength of these. Compounds that are gases at room temperature are all covalent compounds such as CO 2 SO 2 and NH 3 that contain two or more nonmetals.
It is a liquid at room temperature. 1 Write the name of the element that is the farthest to the left and nearest to. After complete evacuation of both chambers valve b is closed and a sample of CO-g is introduced through valve a.
Melting a simple molecular compound requires breaking intermolecular forces like van der Waals forces. Elements that are gases at room temperature are all nonmetals such as He Ar N 2 O 2 and so on. Therefore many of them are liquids or gases at room temperature.
Molecular compounds are chemical compounds that take the form of discrete molecules. They are known as organic solvents. All elemental ionic compounds are solid at room temperature however there is a class of room temperature ionic liquids.

Solved 22 Molecular Compounds Of Low Molecular Weight Tend Chegg Com

6 1 How Compounds Form Pg 210 Compounds A Pure Substance Made Up Of 2 Or More Elements That Are Chemically Combined Ex H 2 O Made Of The Elements Ppt Download
Types Of Chemical Compounds Two Types Of Chemical
What Is A Simple Molecular Compound Which Is Solid At Room Temperature And Insoluble In Water Quora

2 2 Molecular Compounds Pp 61 69 First Some Useful Vocabulary Diatomic Molecules Consist Of Two Atoms Sharing A Covalent Bond Polyatomic Molecules Ppt Download

Chemistry L7 Part 2 Flashcards Quizlet

6 2 Comparing Ionic And Molecular Substances Chemistry Libretexts
0 comments
Post a Comment